Surgical Trials

Perf-Act BreCon: A Prospective Case-control Study to Compare Tissue Perf usion between RetrA ctors and Non-retractors during Immediate Bre ast ReCon struction
Amit Agrawal, Alex Azevado

CLARITY: SurgiCaL educAtion to Reduce IncorrecT care pathwaYs and enhance patient outcomes in right iliac fossa pain

Type B dissections: keeping it “uncomplicated” in a complicated world
Bianca Biersteker, Joost van der Vorst, Jacob Budtz-Lilly

PROTECT: a national perioperative platform trial to improve surgical outcomes
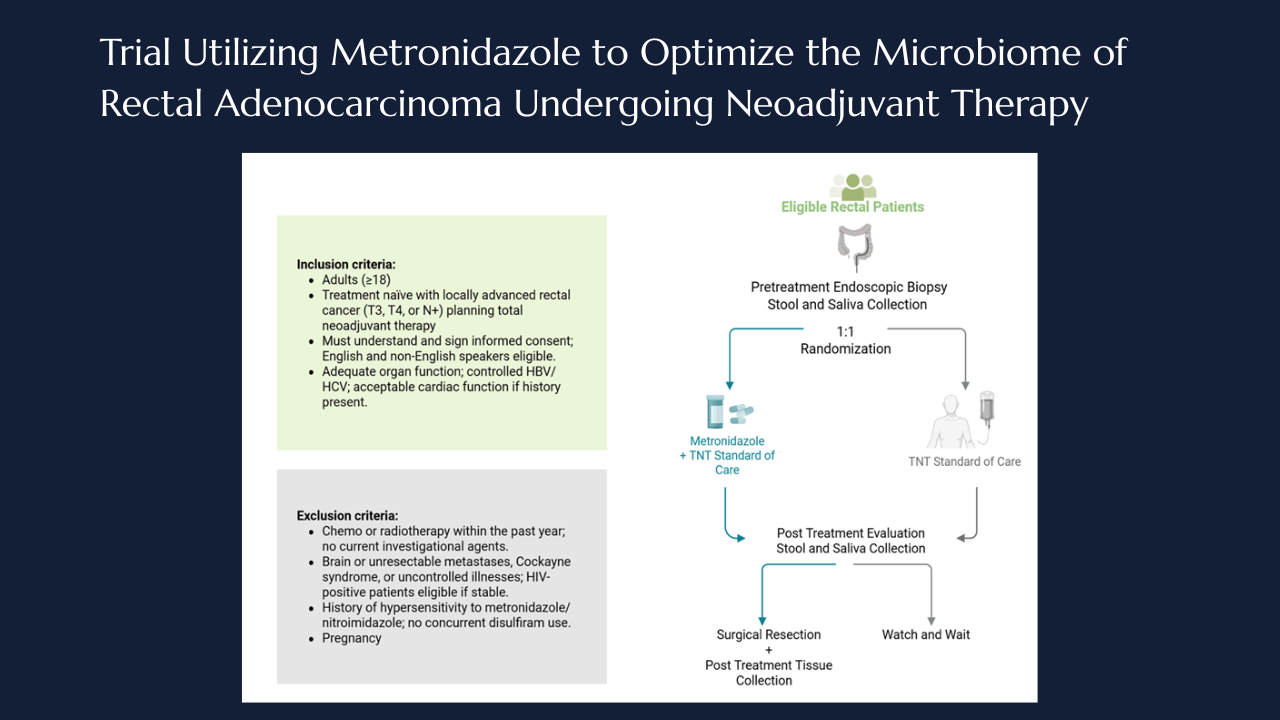
Targeting intratumoral microbiome: the MONARCH Trial takes aim at anaerobes in rectal cancer
Taylor M. Neilson, Laurence P. Diggs, Norman J. Galbraith, Jaganmurugan Ramamurthy, Neal Bhutiani, Ian Z. Hu, Arvind N. Dasari, Michael J. Overman, Scott E. Kopetz, Wei Qiao, Pranoti Sahasrabhojane, Vivian Orellana, Brian K. Bednarski, Montserrat Guraieb-Trueba, Ramy S. Behman, Ashish Damania, Nadim J. Ajami, Yan Wang, Mingxuan Xu, George J. Chang, Jennifer A. Wargo, Susan Bullman, Christopher D. Johnston, Y. Nancy You, Michael G. White

Pain and reward in emergency surgery trials
Mark Edwards
IMPROVE-AD: Randomized trial for uncomplicated Type B Aortic Dissection

THrough knee AMputation’s impact on quality of Life compared to abovE knee ampuTation: THE HAMLET TRIAL
Sophie James , Sean Pymer , Catherine Arundel, Laura Doherty, Tom Davill, George Smith

Closing the treatment gap in knee osteoarthritis — the GEKO trial begins
Dr Raman Uberoi, Dr Anjali Shah, Dr Bhavisha Patel, Dr Loretta Davies, Professor Andrew Price, GEKO study group

THRIVE Trial: Advancing Thromboprophylaxis in Superficial Venous Intervention
Sarah Whittley
The NEON trial: nerve repair vs. alignment for digital nerve injuries
Justin C R Wormald, MRCS, DPhil



.png)





.jpg)




