BJS Academy>Surgical trials>Pain and reward in e...
Pain and reward in emergency surgery trials
Mark Edwards
Consultant & Honorary Professor in Anaesthesia and Perioperative Medicine, Southampton; Co-chief Investigator, CAMELOT trial
8 December 2025
Trials Lower GI
Related articles
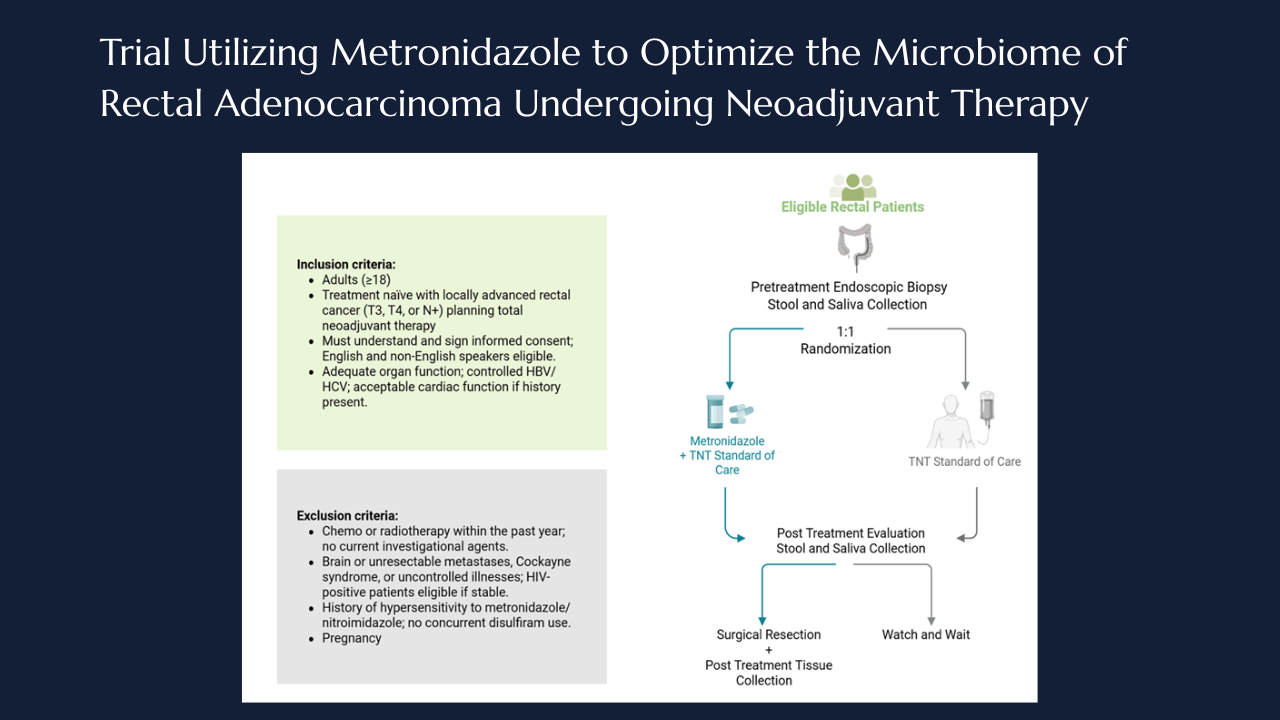
Targeting intratumoral microbiome: the MONARCH Trial takes aim at anaerobes in rectal cancer
Taylor M. Neilson, Laurence P. Diggs, Norman J. Galbraith, Jaganmurugan Ramamurthy, Neal Bhutiani, Ian Z. Hu, Arvind N. Dasari, Michael J. Overman, Scott E. Kopetz, Wei Qiao, Pranoti Sahasrabhojane, Vivian Orellana, Brian K. Bednarski, Montserrat Guraieb-Trueba, Ramy S. Behman, Ashish Damania, Nadim J. Ajami, Yan Wang, Mingxuan Xu, George J. Chang, Jennifer A. Wargo, Susan Bullman, Christopher D. Johnston, Y. Nancy You, Michael G. White
Locally advanced rectal cancer involves multimodality treatment that combines 5-fluorouracil (5-FU) based chemotherapy, chemoradiation and surgical resection. Total neoadjuvant therapy and radiation (TNT) has shown to induce complete tumor regression in up to 30-40% of patients1 and is associated with improved disease free survival.2 Furthermore, patients who attain a complete response to TNT, may be eligible for organ preserving strategies, offering them the potential to avoid radical resection and its attendant morbidity, including possible low anterior resection syndrome, bowel dysfunction, or permanent stoma formation.2-5 Despite its benefits, response to TNT remains markedly heterogeneous with more than half of patients not attaining a complete clinical response.6 The biological determinants underlying this variability remain an area many researchers are working to define. Identifying mechanisms that underlie variable treatment responses may enable novel strategies to enhance tumor regression and expand organ-preservation opportunities.
Emerging data from our group and others has suggested that the intertumoral microbiome may influence the variability in treatment response.7-9 Anaerobic bacteria, particularly Fusobacterium nucleatum8,10, have been associated with resistance to TNT and poor pathologic regression.11 Importantly, the intratumoral microbiome has shown to be dynamic9,12 and can be influenced by variables such as stress, diet, and therapies.13 Models have demonstrated that exposure to agents such as 5-FU or metronidazole can eradicate fusobacterium nucleatum and when eradicated patients had improved outcome.11,12,14,15
Building on these observations, we developed a phase II clinical trial to quantify intratumoral bacterial populations, particularly anaerobes and evaluate the impact of metronidazole administration on these tumoral bacterial populations. Metronidazole, an imidazole antibiotic, acts as a prodrug that is activated under anaerobic conditions, disrupts bacterial DNA synthesis ad metabolism.16,17 This trial aims to determine if selective depletion of anaerobes can be enhanced by the administration of metronidazole.

THRIVE Trial: Advancing Thromboprophylaxis in Superficial Venous Intervention
Sarah Whittley
Superficial venous intervention has transformed the treatment of varicose veins, offering patients minimally invasive solutions with excellent short-term outcomes. Yet, despite technical advances, an important question remains unresolved: Should pharmacological thromboprophylaxis be routinely prescribed to prevent postoperative deep vein thrombosis (DVT)?
The THRIVE trial (THRomboprophylaxis In superficial endovenous interVEntion) is the first randomised controlled study designed to address this uncertainty.1 By generating robust clinical evidence, THRIVE has the potential to influence international practice and shape future guideline recommendations.
Varicose veins are common, affecting up to 45% of the UK population and are associated with reduced physical and mental health-related quality of life.2,3 Symptomatic varicose veins are now routinely treated with endovenous thermal ablation, non-thermal ablation or mechanochemical techniques.4 While effective, these procedures carry a recognised risk of venous thromboembolism (VTE).

Closing the treatment gap in knee osteoarthritis — the GEKO trial begins
Dr Raman Uberoi, Dr Anjali Shah, Dr Bhavisha Patel, Dr Loretta Davies, Professor Andrew Price, GEKO study group
Knee osteoarthritis is one of the most common musculoskeletal conditions, affecting almost one in five people over the age of 45 in the UK — around 4 million people. For many, it means living with chronic pain, limited mobility, and a gradual decline in quality of life. At its most severe, the only effective option is knee replacement surgery.
But what about those who aren’t yet at that stage? Despite trying physiotherapy, anti-inflammatories, and combinations of painkillers, many patients remain stuck in a treatment gap. Their pain is poorly controlled, yet surgery is not an option.
A new approach — genicular artery embolisation (GAE) — might offer hope. The procedure blocks small blood vessels around the knee that are thought to drive inflammation and pain. Early studies have hinted at benefit, but the evidence so far is mixed: one small trial showed no improvement, while another found a modest reduction in pain. Despite this uncertainty, the technique is already being used internationally.
Copied!
Connect

Copyright © 2026 River Valley Technologies Limited. All rights reserved.



.png)





.jpg)



