BJS Academy>Continuing surgical ...>The role of artifici...
The role of artificial intelligence in diagnostic medical imaging and next steps for guiding surgical procedures

Barbara Seeliger MD, PhD, FACS
Digestive Surgeon Institute of Image-Guided Surgery, IHU-Strasbourg, Strasbourg, France Department of Digestive and Endocrine Surgery, University Hospitals of Strasbourg, Strasbourg, France ICube, UMR 7357 CNRS, University of Strasbourg, Strasbourg, France IRCAD, Research Institute Against Digestive Cancer, Strasbourg, France

Alexandros Karargyris PhD
Machine Learning Researcher Institute of Image-Guided Surgery, IHU-Strasbourg, Strasbourg, France ICube, UMR 7357 CNRS, University of Strasbourg, Strasbourg, France

Didier Mutter MD, PhD, FACS, FRSM
Institute of Image-Guided Surgery, IHU-Strasbourg, Strasbourg, France Department of Digestive and Endocrine Surgery, University Hospitals of Strasbourg, Strasbourg, France IRCAD, Research Institute Against Digestive Cancer, Strasbourg, France
Related articles
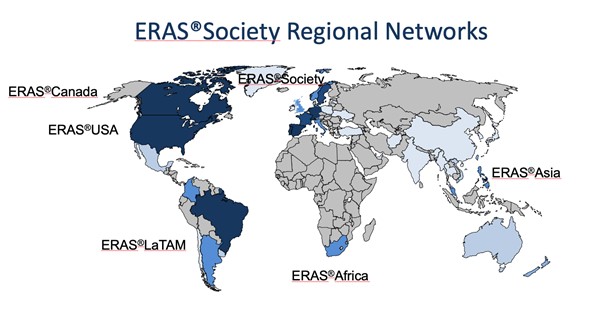
ERAS – yesterday, today and also for tomorrow? The ERAS Society perspective.
Olle Ljungqvist, Ulf Gustafsson, Hans D. de Boer
ERAS so far It is more than 25 years ago that a multimodal approach to recovery after major surgery, called fast track surgery, was first proposed1,2. Combined with laparoscopic surgery it showed that old and frail patients were fit to leave the hospital in two days after major surgery3. Larger follow up studies reported that this could be achieved with fast track surgery alone. This inspired a group of surgeons from Northern Europe to form the Enhanced Recovery After Surgery (ERAS) Study Group in 20014. The members hypothesized that bringing together all potential stress reducing and recovery improving care elements into one program, would enhance recovery after surgery. The first ERAS protocol was published in 20055. But alongside the guideline there was a need to also organize care in a new way to make ERAS fully functioning6 (Table 1). When the guideline was tested a clear relationship was shown with more care elements in the protocol in use and improved outcomes regarding both complications, length of stay and readmissions suggesting that detailed audit would be key7-9.
Robotics surgery
Omar Yusef Kudsi, MD, MBA, FACS
Introduction With multiple advantages over laparoscopic and open surgery, including stereovision, enhanced precision and dexterity, surgeons are transitioning to robotic surgery. Practicing robotic surgeons praise the platform’s improved ergonomics and camera control, advantages that are worth the challenge of overcoming the steep learning curve. Thus, robotics is becoming the cornerstone for advancing the field of minimally invasive surgery. An obvious pattern in the diffusion of cutting-edge technologies is that it starts with one manufacturer – Intuitive Surgical has currently near complete dominance of the robotic surgery market. However, in the future, new robotic platforms will become available. Here we discuss the advantages and challenges with robotic surgery.

Resilience and the modern surgeon
Dr Agnes Arnold-Forster
Military metaphors The place (and some of the problem) of resilience in surgery lies in its origins in the profession’s long historical association with the military. In the nineteenth century, members of the medical professions exploited and elaborated, as historian Michael Brown has put it, “visions of masculinity framed by war, heroism, and self-sacrifice.”1 Clinical practice was conceptualised as a form of warfare against a malevolent enemy and military metaphors were used to refer both to the activities of germs, gangrene, and cancerous tumours, and to the actions of surgeons and physicians. The military metaphor worked on multiple levels. Surgeons were waging war against damage, disability, and disease – inanimate, if deadly foes. Surgeons were also increasingly seen as part of a society-wide conflict between life and death, cures and killers, progress and stagnation. In 1900, Surgeon-Extraordinary to Queen Victoria, Frederick Treves, spoke at the annual meeting of the British Medical Association. His address entitled, ‘The surgeon in the nineteenth century,’ concluded with a flourish, reflecting on the future of surgeon in a passage suffused with military language: “So as one great surgeon after another drops out of the ranks, his place is rapidly and imperceptibly filled, and the advancing line goes on with still the same solid and unbroken front.”2
Copied!
Connect

Copyright © 2026 River Valley Technologies Limited. All rights reserved.



.png)





.jpg)



