BJS Academy>Cutting edge blog>Comment on: Off the ...
Comment on: Off-the-shelf volume replacement in breast-conserving surgery: oxidized regenerated cellulose for upper inner quadrant defects.
Gianluca Franceschini Fondazione Policlinico Universitario Agostino Gemelli IRCCS; Università Cattolica del Sacro Cuore; Department of Woman and Child Health and Public Health; Breast Unit. Rome, Italy.
26 July 2022
Guest Blog Breast
Related articles

Guest post: Mental health and BRCA
Grace Brough, Douglas Macmillan, Kristjan Asgeirsson, and Emma Wilson Division of Epidemiology and Public Health, University of Nottingham Nottingham Breast Institute, Nottingham University Hospitals NHS Trust
Exploring psychological consequences for BRCA+ women in the post-Covid era
Whilst the global female population has a 12.5% overall lifetime risk of developing breast cancer and a 1.3% risk of ovarian cancer (Howlader et al), the risk for those with a pathogenic BRCA1 or BRCA2 mutation is 60-70% and 10-20% respectively (van Egdom et al). BRCA1 mutation carriers have a particularly high incidence of triple-negative breast cancer (TNBC) (Greenup et al) for which treatment options are more limited and always include chemotherapy (Bianchini et al; Collignon et al).
In the NHS, asymptomatic women with at least a 10% estimated chance of having a BRCA mutation are offered testing (NICE). Knowing you are at high risk of breast cancer and the increased likelihood of TNBC is a well-documented cause of anxiety (Wenzel et al) and many women describe having a BRCA gene mutation as living with a ‘ticking time bomb’. Bilateral mastectomy with or without reconstruction is the only proven method of drastically decreasing risk and can improve quality of life (McCarthy et al) and decrease anxiety (Rebbeck et al) for correctly selected cases, despite its potential negative outcomes (Gahm et al).

Guest blog: Invasive lobular cancer of the breast remains a surgical challenge
U Narbe, P -O Bendahl, M Fernö, C Ingvar, L Dihge, L Rydén
Invasive lobular breast cancer is the second most common histological subtype and constitutes around 10-15% of all breast cancer. It is recognized by a peculiar diffuse growth pattern and disseminated spread of cancer cells contributing to diagnostic delay and difficulties in detecting the primary tumors by mammography and nodal metastasis by axillary ultrasound. Consequently, although invasive lobular breast cancer mainly displays a favorable luminal A molecular subtype, it is often diagnosed at a higher stage than invasive cancer of no special type. Despite these characteristics, histopathological subtype is not accounted for in Clinical Guidelines, in contrast to the molecular subtypes. In the BJS publication “The St. Gallen 2019 Guidelines understage the Axilla in Lobular Breast Cancer: a Population-Based Study” by Ulrik Narbe and co-authors, we have elaborated on the consequences for patients with invasive lobular cancers if completion axillary clearance would be omitted in patients with 1-2 nodal metastases.
For the breast surgeon, lobular cancer poses a challenge to surgery of the breast and to the axilla. Large tumor size and diffuse infiltrating margins are associated with an increased risk of positive margins after breast conserving surgery and in breast centers where MRI is available, complementary imaging is recommended ahead of primary surgery. Still a larger proportion of patients (66%) with lobular cancer undergo a mastectomy compared to other histological subtypes (43%) according to our registry study, supporting previous publications. So the dogma of de-escalating breast surgery from mastectomy to partial mastectomy in lobular cancer is challenging, keeping in mind that positive resection margins is a mandatory quality criteria in most centers pushing the choice of initial surgery towards upfront mastectomy.
Axillary surgery is moving towards de-escalation of surgical interventions for patients with 1-2 sentinel node metastases after the results of the Z0011 trial have been implemented worldwide and clearly supports that omission of completion axillary dissection is non-inferior provided the inclusion criteria for the trial is fulfilled. The Z0011 criteria for omission of completion axillary dissection is restricted to patients undergoing breast-conserving surgery, and is thus not applicable for lobular cancers operated by mastectomy. The St. Gallen 2019 guidelines extended the indication for abstaining completion axillary dissection to all patients of any tumor size irrespective of type of breast surgery. In addition, the guidelines recommended that the decision on adjuvant chemotherapy for luminal A-like tumors should include nodal staging and patients with 4 or more nodal metastasis (nodal stage II) were recommended adjuvant chemotherapy. For these patients there is hitherto no data on the role of genomic tests for risk stratification until the results of OPTIMA trial are presented. In this paper, we present data from the National Swedish Quality Registry with prospectively collected data retrieved from the period when a completion axillary clearance for patients with sentinel node metastases was recommended. We found a strong association between invasive lobular breast cancer and nodal stage II disease independent of other determinants such as tumour size.
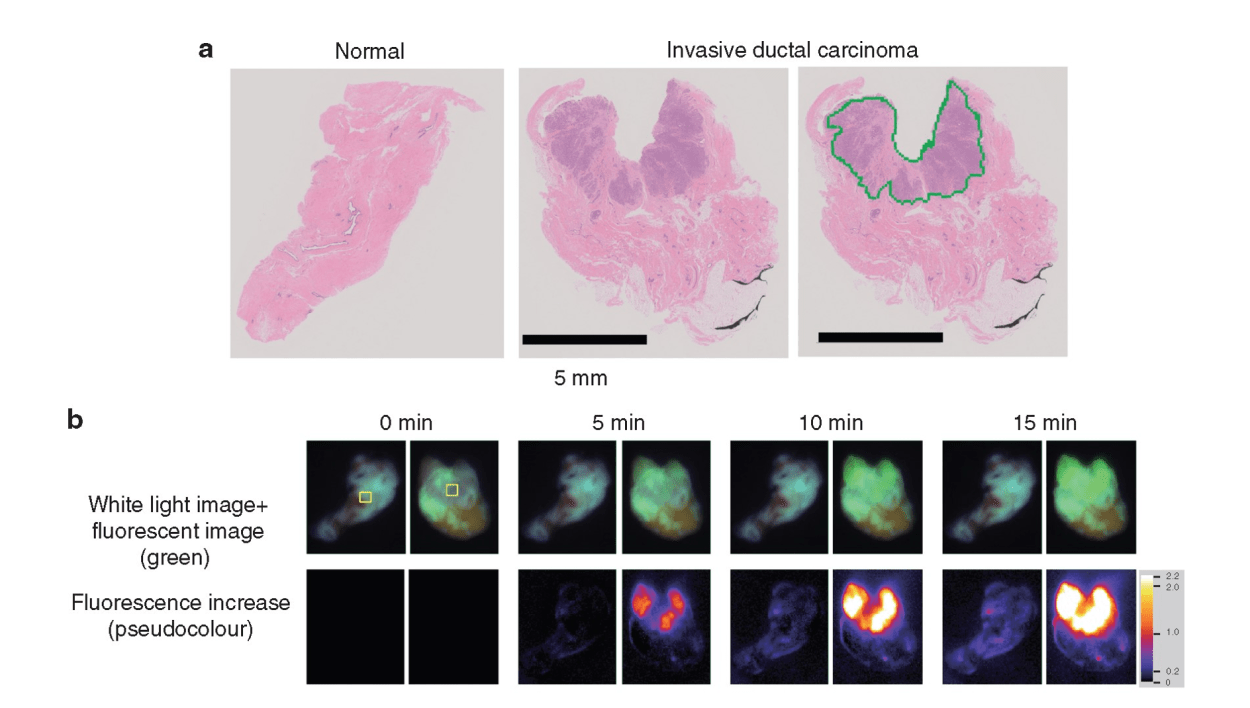
Guest blog: a novel fluorescence technique for detecting breast cancer
Hiroki Ueo, Department of Surgery and Sciences, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; Ueo Breast Cancer Hospital, Oita, Japan
Breast cancer is the most common cancer in women, and its incidence continues to increase worldwide. From the patient’s perspective, breast conserving surgery (BCS) with radiation achieves a balance between a satisfactory cosmetic result and a low recurrence rate. Although it has been established as a routine surgery, surgeons need to be careful about positive surgical margins. Remnant cancer cells in the preserved tissue increase the risk of recurrence. Therefore, a positive margin on postoperative pathology warrants additional surgery. In these cases, the additional treatment harbours unexpected outcomes, including physical, mental, cosmetic, and economic burden on the patients.
To avoid the additional operation, pathological evaluation using an intraoperative frozen section is conducted. It is the most reliable method to prevent misdiagnosis and to achieve clear surgical margins. However, this conventional method is time consuming and costly. Moreover, it is dependent on the skill and experience of the pathologists and personnel, and it requires space for preparation of the frozen sections. Therefore, only a limited number of samples are examined to save time and resources. An alternative, rapid, and reliable technique to detect cancer in surgical margins enables simultaneous testing, leading to a reduced false negative rate of local recurrence incidence. In addition, pathologists can focus on the definitive diagnosis using permanent paraffin sections because it is difficult to make a diagnosis based on intraoperative frozen sections without pathological architecture. Pathologists only need to make an intraoperative diagnosis when the specimen cannot be evaluated via the fluorescence procedure. Thus, it is important to enhance the rapid fluorescent detection of breast cancer during surgery. To address these diagnostic issues, Prof. Urano invented chemical reagents (gamma-glutamyl hydroxymethyl rhodamine green [gGlu-HMRG]) that quickly fluoresce by reacting with an enzyme (gamma-glutamyl transferase [GGT]), overexpressed in cancerous tissues. It exhibits strong fluorescence a few minutes after reacting with GGT in vitro. A gGlu-HMRG solution is applied to the surgical margins to recognize cancer cells as green fluorescence intraoperatively. A previous study in 2015 documented the ability of this reagent to mark cancerous tissues in surgical breast tissues. Furthermore, this reagent did not interfere with the pathological examination, while the frozen section analysis tissues were difficult to reuse as formalin-fixed and paraffin-embedded permanent pathological specimens.
The clinical utility of this technique was examined. The results were published in the British Journal of Surgery. Since the initial report in 2015, a more feasible and reproducible sample preparation protocol has been developed. Then, a dedicated apparatus, including a built-in camera, software programme, and multiple sample wells, was developed. This system automatically measured and analyzed the increase in fluorescence of multiple samples simultaneously. Then, the increase in fluorescence of gGlu-HMRG, applied to breast tissues, was measured in four different institutes. The sample tissues were examined by four pathologists independently. These pathologists diagnosed the samples without knowing the background information of the patients. The clinical utility of the current fluorescent procedure was evaluated by comparing the fluorescence data and the pathological diagnosis.
Copied!
Connect

Copyright © 2025 River Valley Technologies Limited. All rights reserved.








.jpg)



