BJS Academy>Cutting edge blog>Plain English Summar...
Plain English Summary: How the first COVID‐19 wave affected UK vascular services
9 October 2020
Guest Blog Vascular
Related articles
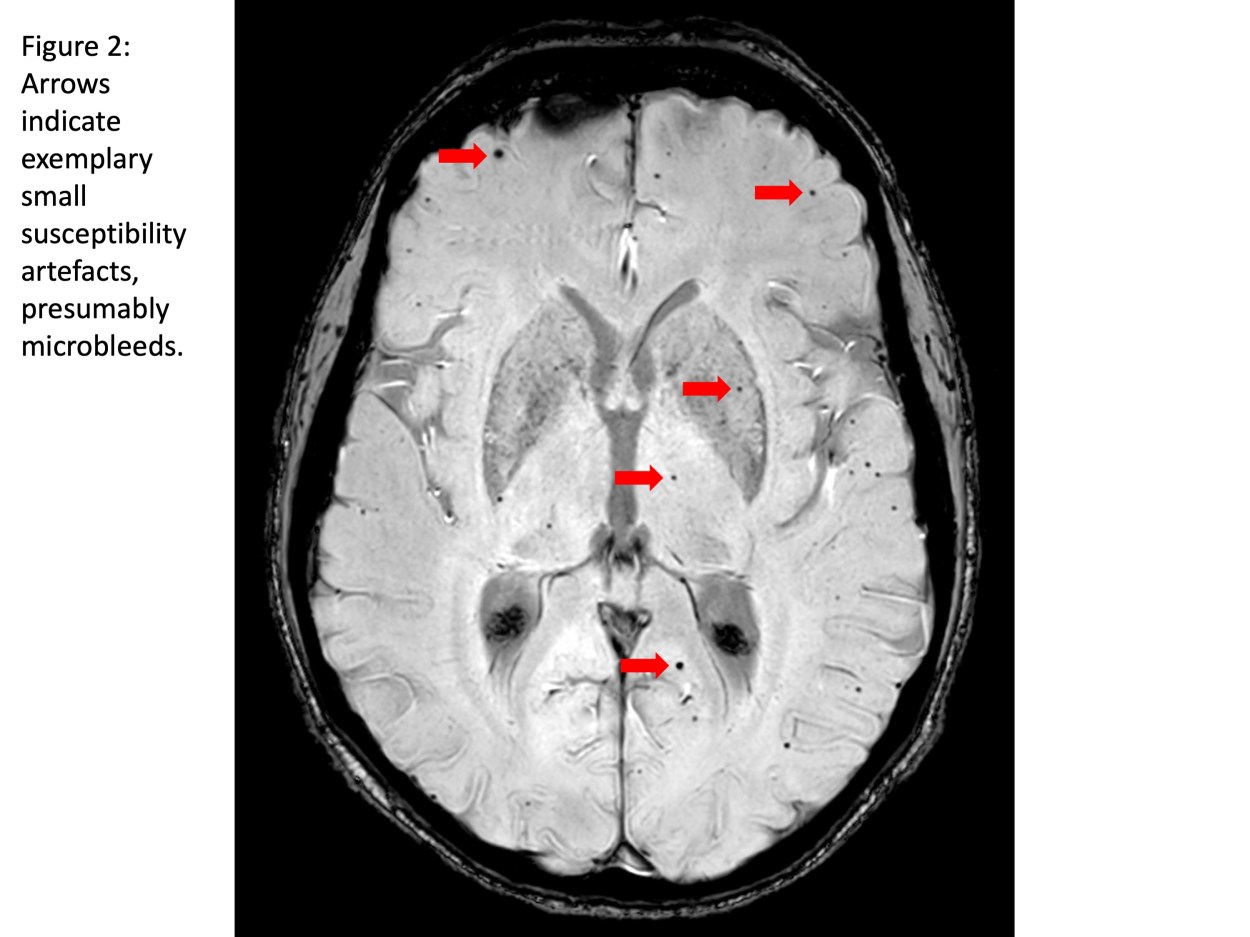
Guest blog: Cerebral microbleeds following thoracic endovascular aortic repair
W. Eilenberg a,b**, M. Bechstein d**, P. Charbonneauc, F. Rohlffs a, A. Eleshra a, G. Panuccio a, J. Bhangu b, J. Fiehler d, R. Greenhalgh e, S. Haulon c, T. Kölbel a*
a German Aortic Center, Department of Vascular Medicine, University Heart & Vascular Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany
b Department of General Surgery, Division of Vascular Surgery, Medical University of Vienna, Vienna, Austria
c Centre de l’Aorte, Hôpital Marie Lannelongue, Groupe hospitalier Paris Saint Joseph, Université Paris Saclay, France
d Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
e Vascular Surgical Research Group, Imperial College, London, UK.
** both authors contributed equally
E-mail:
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Stroke and cerebral damage are frequent findings after thoracic endovascular aortic repair (TEVAR) with a postoperative clinical stroke rate of 3-4% and silent brain infarcts (SBI) in about 80%.(1-4) However, the mechanism of stroke and subclinical cerebral damage in TEVAR is under-investigated. Current clinical research-efforts such as the STEP-registry (strokes from thoracic endovascular procedures) aim to better understand incidence of, and risk factors for stroke and cerebral damage after TEVAR, and to develop strategies for prevention.(5, 6) More than 60% of patients undergoing arch-TEVAR were reported to have SBIs on diffusion weighted magnetic resonance imaging (DW-MRI) despite protective efforts such as carbon dioxide (CO2) flushing of the endografts.(5, 6) The aim of the current study is to examine the occurrence of CMBs in patients after TEVAR within the STEP-registry and to evaluate their association with patient- and procedural factors.
Ninety-one patients treated with TEVAR in proximal landing zone (PLZ) 0-3 from September 2018 to January 2020 at the German Aortic Center (Hamburg, Germany) and Marie Lannelongue Hospital (Paris, France) were included in the study.(5) The location and number of CMBs were identified and analyzed with regards to procedural aspects, clinical outcome and Fazekas-score as indicator of preexisting vascular leukoencephalopathy.
Insurance status may impact on survival from malignant cardiac tumours
Mohamed Rahouma, Massimo Baudo, Shon Shmushkevich, David Chadow, Abdelrahman Mohamed, Mario Gaudino, Roberto Lorusso
Cardiac neoplasms may develop as either a primary malignant disease or as metastases from an extra-cardiac location1. The incidence of cardiac malignancies is very low and only about 10% of primary cardiac tumours are malignant2. The most frequent benign cardiac tumour is myxoma, while the majority of primary malignant cardiac tumors (PMCTs) are sarcomas, consisting of various histological subtypes3. In general, an aggressive biological behaviour is typical of primary cardiac tumours4, and is associated with tumour size and location5. Survival outcomes remain poor even if detected early and aggressive surgical resection is the main primary curative option6,7. Survival is only around 10% at one year for tumours managed without surgery8,9. To date, the combination of surgery with systemic chemotherapy remains the best treatment for malignant cardiac tumours9. No consensus currently exists concerning the value of peri-operative chemotherapy and radiotherapy10,11,12. Palliative chemotherapy should be considered for patients with inoperable or metastatic disease.
In the current study we aimed to assess long-term overall survival (OS) differences based on insurance status in patients with malignant cardiac tumours using the National Cancer Database (NCDB). The NCDB is an oncology database that is sponsored by the American Cancer Society and the American College of Surgeons. We included data from the NCDB from 2004 to 2017. Overall survival and operative mortality were the primary and secondary outcomes, respectively.
Our study cohort included 699 patients that were stratified by insurance type: 412 (58.9%) had private insurance, 243 (34.8%) had governmental insurance and 44 (6.3%) were uninsured. Overall, operative mortality was 8.5%: 11.1% in the Uninsured, 2.6% in Medicaid, 13.9% in Medicare and 7% in Private Insurance/Managed Care groups, respectively (p=0.09). Histopathological details of the included patients can be seen in Table 1. Angiosarcoma, leiomyosarcoma and fibrosarcoma were the most prevalent variants in this cohort. Figure 1 describes the type of insurance over the considered timeframe. Private insurance/manages was always the most common form of insurance.

PelvEx 2024
John T. Jenkins1, Paul Sutton2
1. Consultant Colorectal Surgeon
Chair of Surgery
Lead Complex & Recurrent Cancer Service,
St. Mark's Hospital, London
Imperial College, London
Surgeon to the Royal Household
2. Consultant Colorectal, Pelvic, and Peritoneal Surgeon
Colorectal and Peritoneal Oncology Centre
The Christie NHS Foundation Trust
Chair ACPGBI Advanced Malignancy Subcommittee
DOI: https://doi.org/10.58974/bjss/azbc052
PelvEx 2024 was hosted and organized in London by the ACPGBI Advanced Malignancy Subcommittee and UKPEN. The Royal Institution provided the perfect venue, blending history with excellent facilities which ensured the meeting was a tremendous success. Nearly 300 delegates attended from all over the world, with this year’s meeting having a truly multi-disciplinary programme.
The first day included sessions on “R0 resection – How important is it really”, “How much is too much in exenteration surgery”, and “Mastering morbidity”. In addition, sessions on the “Consequences of exenteration surgery” including patient and nursing experiences, as well as discussion about sex and intimacy, and the psychological consequences of surgery. The day finished with a “Consultants Corner- Grey Heads on Green Shoulders” session discussing a number of diverse challenges encountered at different stages in clinical practice .
The second day began with the inaugural trainee prize presentation session for the now coveted Brunschwig Prize. A number of high quality abstracts were received and the six best (linked below this blog) were invited to deliver oral presentations. The quality was exceptional. This session was followed by sessions on “Research progress” and “Surgical techniques”, followed by an afternoon where the “Oncologists have their say” culminating with a “Global MDT”. The conference was live streamed to China and recorded for posterity.
Copied!
Connect

Copyright © 2026 River Valley Technologies Limited. All rights reserved.



.png)





.jpg)



