BJS Academy>Cutting edge blog>Impact of postoperat...
Impact of postoperative chemotherapy on survival for oesophagogastric adenocarcinoma after preoperative chemotherapy and surgery.
Tim J. Underwood, University of Southampton, Southampton, United Kingdom
22 November 2022
Guest Blog Upper GI
Related articles

Management of Crohn’s Disease. The shiny medical sports car or the worn out surgical banger?
Professor Steven R Brown, Sheffield Teaching Hospitals. Based on the BJS Lecture at ACPGBI 2020
Professor Steven R Brown, Sheffield Teaching Hospitals.
Based on the BJS Lecture at ACPGBI 2020
A success story
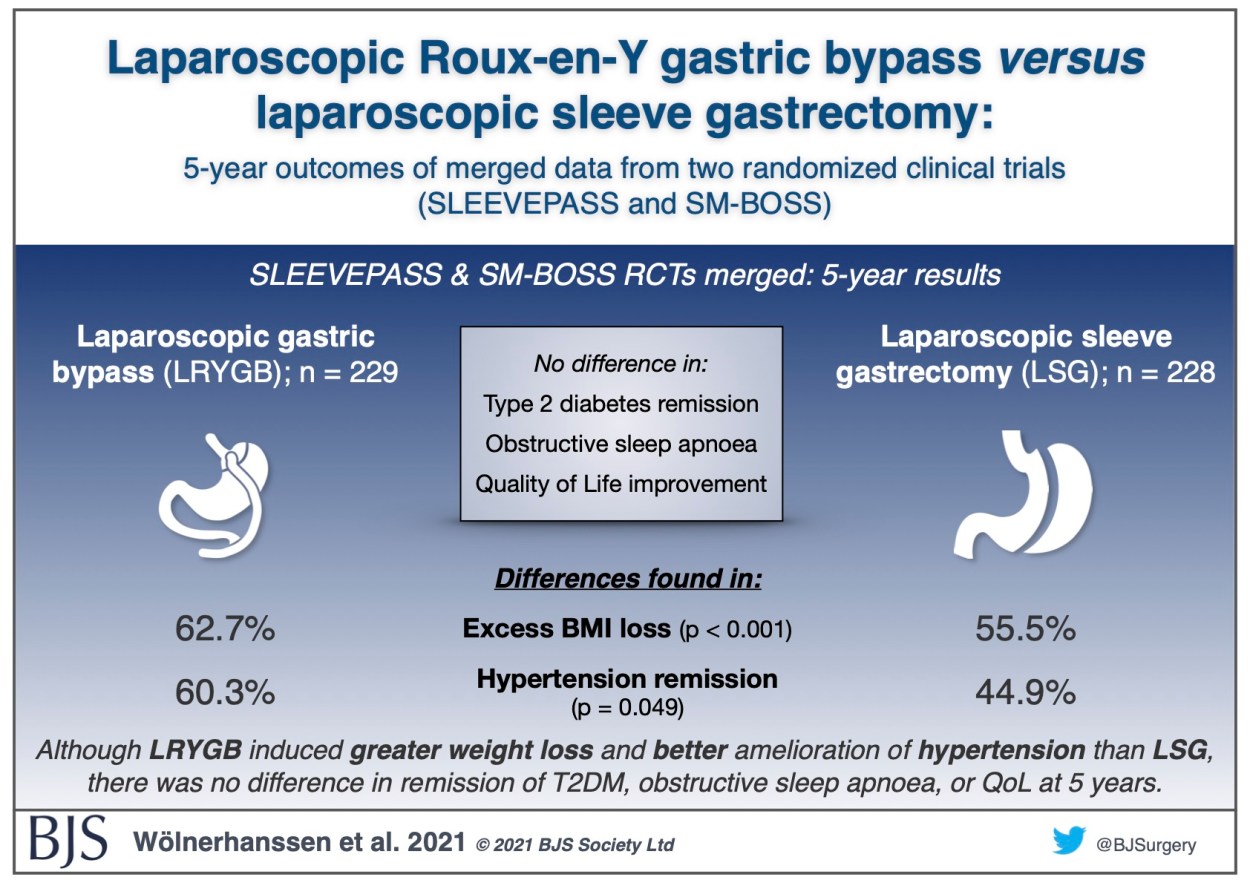
Visual abstract: Merged SLEEVEPASS and SM-BOSS trials
Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS)
Recently published as open access in BJS, the 5-year results of the merged Finnish SLEEVEPASS and Swiss SM-BOSS randomised controlled trials comparing laparoscopic sleeve gastrectomy with laparoscopic Roux-en-Y gastric bypass shows that Roux-en-Y led to greater weight loss and better control of hypertension than sleeve gastrectomy, with no difference in outcomes for type 2 diabetes, obstructive sleep apnoea, or quality of life. More details can be found in the paper.
Guest blog: Keyhole versus open surgery for oesophageal cancer
B. P. Müller-Stich, P. Probst, H. Nienhüser, S. Fazeli, J. Senft, E. Kalkum, P. Heger, R. Warschkow, F. Nickel, A.T. Billeter, P. P. Grimminger, C. Gutschow, T. S. Dabakuyo-Yonli, G. Piessen, M. Paireder, S. F. Schoppmann, D. L. van der Peet, M. A. Cuesta, P. van der Sluis, R. van Hillegersberg, A. H. Hölscher, M. K. Diener, T. Schmidt
Minimally invasive resection of esophageal cancer might be less traumatic than open resection and has the potential to reduce complications and even improve survival. In contrast, oncological radicality might be negatively affected by the minimal-invasive approach. The aim of this BJS study was to generate 1A level of evidence on the question whether a minimally invasive approach for oncological esophagectomy is advantageous. A systematic literature search was performed and exclusively randomized-controlled trials (RCTs) comparing minimally invasive to open oncological esophagectomy were included in a meta-analysis.
Among 3219 articles six RCTs (four trials from Europe, two from Asia) were found including 822 patients. Survival data and short-term postoperative outcome data was analyzed. From the four European trials (Biere et al. Lancet 2012; Paireder et al. Eur Surg 2018; van der Sluis et al. Ann Surg 2019; Mariette et al. NEJM 2019) individual patient data was retrieved to analyze survival according to the different surgical approaches. Overall survival (56% minimally invasive) vs. (52% open) and disease-free survival (54% vs. 50%) after three years were comparable. Strikingly, the risk of postoperative complications was significantly reduced to one third in the minimal invasive group mainly due to the reduction of pulmonary complications and, in particular, pneumonia. Other parameters, especially those indicating oncological quality of the resection as number of harvested lymph nodes, did not differ between the two groups while the operation time was shorter in the open group. There was no significant difference in the rate of anastomotic leakage, length of stay in the intensive care unit or in the hospital and in the perioperative mortality while total blood loss was lower in the minimal invasive group.
As this meta-analysis included only high-quality randomized controlled trials, it generates high level evidence for the perioperative advantages of minimal invasive esophagectomy. The minimally invasive approach significantly reduces the risk of complications compared to open surgery and does not impair long-term oncological outcome. It should therefore be the preferred approach for cancer-related oesophagectomy.
Copied!
Connect

Copyright © 2025 River Valley Technologies Limited. All rights reserved.








.jpg)



