BJS Academy>Cutting edge blog>European Hernia Soci...
European Hernia Society guidelines on management of rectus diastasis
5 October 2021
Guest Blog Hernia
Related articles
Guest blog in plain English: Hernias in children
Nathalie Auger, Francesca del Giorgio, Annie Le-Nguyen, Marianne Bilodeau-Bertrand, Nelson Piché University of Montreal Hospital Research Centre, Montreal, Quebec, Canada
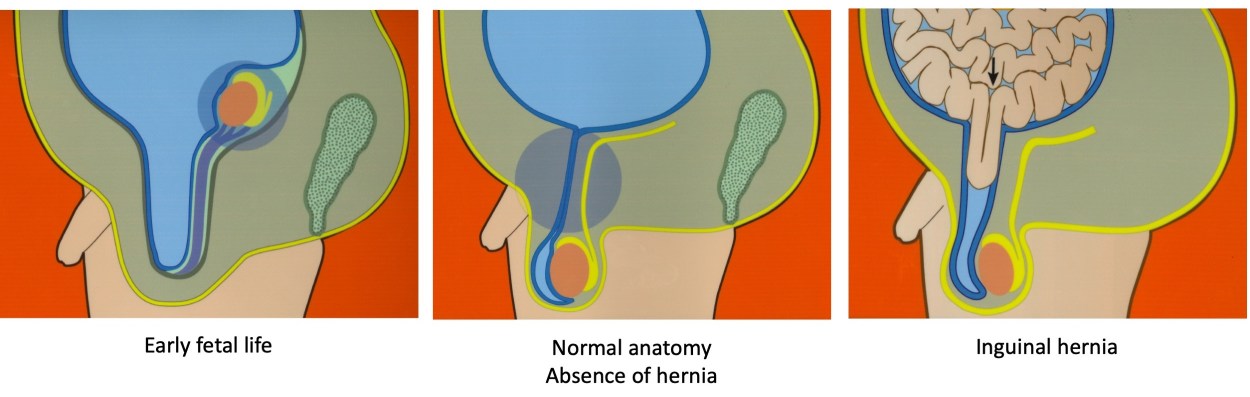
Are women who have inguinal hernias more likely to have a child with an inguinal hernia? Have you ever wondered why a child may develop an inguinal hernia? Inguinal hernias affect about 4% of children1,2, but the reasons why are very unclear. An inguinal hernia occurs when intestinal tissues push through a weak spot in the abdominal wall. Most children with inguinal hernias are thought to have developed this disorder while still in their mother’s womb1,3. Yet there has been little attention to the possibility that the characteristics of mothers could predict which children will develop inguinal hernias while growing up.
We studied whether women who were previously diagnosed or treated for an inguinal hernia were more likely to have a child with an inguinal hernia. To test our research question, we analyzed the health status of 795,590 children from the province of Quebec, Canada4. We collected information from their mothers including her age, pregnancy complications, diseases she may have had in the past, and whether she was ever treated for an inguinal hernia. We closely followed her child over time to find out if the child was ever hospitalized for an inguinal hernia between birth and 13 years of age. We used regression methods to determine how characteristics of the mothers were associated with the chance of having an inguinal hernia in the child, and made sure that we controlled for confounders that could lead to incorrect measurements.
Our findings were very enlightening. We confirmed that mothers with a history of inguinal hernia were more likely to have a child who develops an inguinal hernia. But we found that the risk was higher for daughters than sons. Girls whose mothers had an inguinal hernia were 5 times more likely to themselves have an inguinal hernia. Additionally, mothers with connective tissue disorders such as rheumatoid arthritis or lupus were more likely to have sons with inguinal hernias. The figure illustrates just how strong some of the associations were.
Parastomal hernia: Quality of life
Imran Mohamed, Rhiannon L Harries
The recent article1 outlined the techniques used in contemporary management of parastomal hernia. Reported outcomes are centred around surgical site complications and recurrence. There is, however, a huge burden placed upon the patient who ultimately has to manage the physical as well as psychological stresses associated with a parastomal hernia. There is growing interest in developing optimal management strategies that take into account patient reported outcomes, that do not simply focus on clinical determinants such as successful operative repair.
Patients with parastomal hernia have worse physical functioning, experience more pain and have lower general health as well as experiencing feelings of negative body image, compared to patients without parastomal hernia2,3. Body image encompasses perceptions, emotions and attitudes a person holds with regards to their own body3. Interventions can be designed to improve perceptions of body image, as well as levels of physical activity.
The Hernia Active Living Trial (HALT) aims to evaluate the effect of increased physical activity on the risk of parastomal hernia development3. Increased levels of physical activity may contribute towards improving overall perceptions of body image and also quality of life.

PelvEx 2024
John T. Jenkins1, Paul Sutton2
1. Consultant Colorectal Surgeon
Chair of Surgery
Lead Complex & Recurrent Cancer Service,
St. Mark's Hospital, London
Imperial College, London
Surgeon to the Royal Household
2. Consultant Colorectal, Pelvic, and Peritoneal Surgeon
Colorectal and Peritoneal Oncology Centre
The Christie NHS Foundation Trust
Chair ACPGBI Advanced Malignancy Subcommittee
DOI: https://doi.org/10.58974/bjss/azbc052
PelvEx 2024 was hosted and organized in London by the ACPGBI Advanced Malignancy Subcommittee and UKPEN. The Royal Institution provided the perfect venue, blending history with excellent facilities which ensured the meeting was a tremendous success. Nearly 300 delegates attended from all over the world, with this year’s meeting having a truly multi-disciplinary programme.
The first day included sessions on “R0 resection – How important is it really”, “How much is too much in exenteration surgery”, and “Mastering morbidity”. In addition, sessions on the “Consequences of exenteration surgery” including patient and nursing experiences, as well as discussion about sex and intimacy, and the psychological consequences of surgery. The day finished with a “Consultants Corner- Grey Heads on Green Shoulders” session discussing a number of diverse challenges encountered at different stages in clinical practice .
The second day began with the inaugural trainee prize presentation session for the now coveted Brunschwig Prize. A number of high quality abstracts were received and the six best (linked below this blog) were invited to deliver oral presentations. The quality was exceptional. This session was followed by sessions on “Research progress” and “Surgical techniques”, followed by an afternoon where the “Oncologists have their say” culminating with a “Global MDT”. The conference was live streamed to China and recorded for posterity.
Copied!
